Abstract of my dissertation:
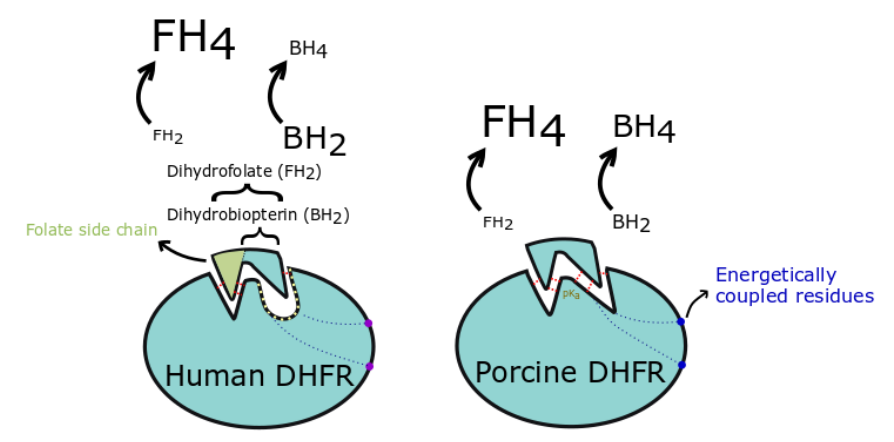
Tetrahydrobiopterin (BH4) is a key cofactor of endothelial nitric oxide synthase (eNOS) that drives formation of nitric oxide (NO), rather than superoxide, and thus plays an important role in endothelial cell function and blood pressure regulation. BH4 can be recycled by dihydrofolate reductase (DHFR), which seems to be important for BH4 bioavailability in some species. A previous study indicated that BH4 recycling efficiency (particularly the binding affinity) is significantly greater in porcine endothelial cells than in human endothelial cells, but the mechanisms were unclear. Here, we investigated the underlying mechanism(s) of enhanced BH4 recycling efficiency in porcine cells. We found that purified recombinant porcine DHFR exhibits significantly higher affinity for BH2 substrate than recombinant human DHFR. We next tried to identify species-specific differences in DHFR structure that contribute to the high affinity of porcine DHFR for BH2. We introduced 4 targeted mutations within or outside of the active site of human DHFR, replacing the human amino acid residues with the corresponding porcine residues (F32Y/R33K/G70D/K55R). Human DHFR affinity for BH2 was improved by the F32Y/R33K and K55R mutation, but was still weaker than porcine DHFR. The studied mutations caused unexpected differences in the catalytic activity of DHFR, particularly for dihydrofolate (FH2) substrate. Based on the 3D structure of DHFR, we discuss how the targeted amino acid substitutions could alter DHFR-substrate and DHFR-cofactor hydrogen bond formation and change the pKa of molecules in the substrate and DHFR structure. Taken together, our findings identify F32Y/R33K as an important element in DHFR binding affinity for BH2 substrate, and suggest additional more distal amino acid differences (K55R in our study) also likely play a role in enhanced porcine DHFR activity.